Shocking decisions in AFib care
Trials’ results highlight the risks and benefits of oral anticoagulation, the superiority of dual direct cardioversion in patients with obesity and the potential for a novel factor XI inhibitor to reduce bleeding in patients with high risk of stroke.
Investigators in four trials revealed results during their Late-Breaking Science Session on Sunday. They found:
- Patients with subclinical AFib may benefit from oral anticoagulation.
- Oral anticoagulation increased the risk of bleeding in patients with atrial high-rate episodes.
- Dual direct cardioversion restored sinus rhythm in more patients with obesity and AFib.
- Novel factor XI/Xa inhibitor holds promise as a next-generation anticoagulant.
Consider oral anticoagulant treatment for subclinical AFib
Current guidelines are uncertain about treating subclinical atrial fibrillation (SCAF) with oral anticoagulation. These short-lasting asymptomatic episodes of AFib, detected by long-term continuous monitoring by implanted devices, are common among patients with pacemakers and defibrillators and those over 65 with cardiovascular risk factors.
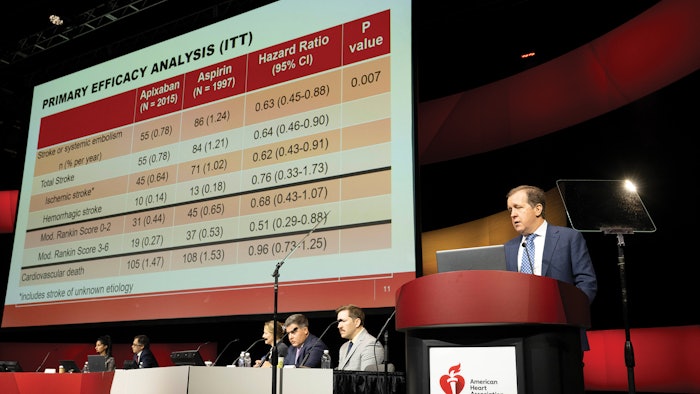
The presence of SCAF is associated with an increased risk of stroke. However, the absolute risk is lower than observed with traditional clinical AFib. The results of Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Subclinical AFib, the ARTESIA trial, suggest a benefit to treat.
The international trial enrolled 4,012 people with SCAF lasting six minutes to 24 hours, detected by an implanted pacemaker, defibrillator or cardiac monitor, and who had additional stroke risk factors. Participants were randomized in a double-blind, double-dummy fashion to Apixaban 5 mg twice daily or 2.5 mg twice daily if participants met criteria for dose reduction, or aspirin 81 mg once daily, for an average of four years.
The mean CHA2DS2-VASc score for atrial fibrillation stroke risk was 3.9±1.1 and the median (25th, 75th) duration of their longest SCAF episode was 1.47 (0.2-4.95) hours.
Apixaban demonstrated a 37% statistically significant reduction in stroke risk, from a rate of 1.24% per year, with a 49% reduction in disabling or fatal strokes, with a Modified Rankin Score of 3-6. The rate of major bleeding was higher for patients receiving apixaban (HR=1.80), with a risk of 1.7% per year.  Jeff Healey, MD
Jeff Healey, MD
“ARTESIA showed that Apixaban reduces stroke compared to aspirin; in particular, it reduces severe stroke. However, you do pay a price with an excess of major bleeding,” said Jeff Healey, MD, senior scientist with the Population Health Research Institute and professor of medicine at McMaster University in Hamilton, Ontario, Canada.
However, Apixaban did not result in any excess of the severe components of major bleeding, including bleeding in the brain, fatal bleeding or bleeding requiring transfusion, Dr. Healey said.
Overall, “we should consider oral anticoagulant treatment in patients with background subclinical atrial fibrillation,” he said. The study will be published in the New England Journal of Medicine following the presentation.
Oral anticoagulation is not effective in patients with atrial high-rate episodes ≥24 hours
Oral anticoagulation increased bleeding in patients with atrial high-rate episodes (AHRE) ≥24 hours, according to Efficacy and Safety of Anticoagulation With Edoxaban in Patients With AHRE Durations ≥24 Hours.
The study was a subanalysis of NOAH-AFNET 6, which found that oral anticoagulation with edoxaban did not reduce the primary outcome of stroke, systemic embolism or cardiovascular death compared to no anticoagulation in patients with AHRE with at least one stroke risk factor (age ≥65). But it increased the risk of major bleeding.
It is the first report on a randomized comparison of oral anticoagulation and placebo in patients with device-detected AHRE ≥24 hours.
The subanalysis included 259/2389 (11%) patients with AHRE ≥24 hours (77.5±6.6 years old, 37% women, median CHA2DS2-VASc score 4). Clinical characteristics did not differ between participants with AHRE ≥24 hours and those with shorter AHRE, except for more AHRE per patient.
Over a median follow-up of 1.8 years, the primary outcome of stroke, systemic embolism or cardiovascular death occurred in 9/132 patients with AHRE ≥24 hours with anticoagulation (4.3% patient/year) compared to 14/127 patients (6.9% patient/year) treated without anticoagulation. Nina Becher, MD
Nina Becher, MD
Among patients with AHRE ≥24 hours with anticoagulation, ischemic stroke occurred in 2/132 patients (0.95% patient/year) and in 2/127 patients with placebo (0.97% patient/year). AHRE duration did not interact with the randomized treatment (P(interaction)=0.65). Compared to placebo, anticoagulation increased the risk of major bleeding. The overall stroke rate was low (1% patient/year) in patients with AHRE ≥24 hours with and without anticoagulation despite multiple clinical stroke risk factors and a median AHRE duration >2 days (53 hours). Patients with AHRE lasting ≥24 hours developed more atrial fibrillation compared to those with AHRE lasting <24 hours, detected by routine ECGs every six months.
“In this subanalysis, patients with AHRE ≥24 hours and multiple stroke risk factors, oral anticoagulation increased bleeding without a clear signal of reducing the primary outcome of stroke, systemic embolism and cardiovascular death or the secondary outcome of ischemic stroke compared to placebo,” said Nina Becher, MD, cardiologist at University Medical Center Hamburg-Eppendorf and the study’s co-investigator.
“Considering the balance between anticoagulation-induced bleeding and stroke prevention, our findings suggest that patients with AHRE ≥24 hours can be managed without anticoagulation until the presence of atrial fibrillation is verified through an ECG.”
Dr. Becher noted that patients with AHRE ≥24 hours often develop ECG-documented AFib over time, underscoring the importance of regular ECG monitoring.
Further research is needed to identify patients with AHRE who are at high risk of stroke and determine how to balance the risk of major bleeding in these complex patients. The study will be published in the European Heart Journal following the presentation.
Dual cardioversion more effectively and safely restored sinus rhythm in patients with obesity
Dual cardioversion (dual-DCCV) — in which two sets of defibrillator pads simultaneously deliver two 200 Joule shocks totaling 400J — is safe and more effective in restoring sinus rhythm in patients with AFib, according to Efficacy and Safety of Dual Direct Current Cardioversion Versus Single Direct Current Cardioversion as an Initial Treatment Strategy in Obese Patients With AFib.
External direct current cardioversion, using a single set of defibrillator pads (single-DCCV), is a common treatment to restore sinus rhythm in patients with AFib. Still, it’s estimated to fail in 20% to 25% of patients with obesity, even with biphasic waveforms at maximal energy output.
The three-year, multicenter, prospective controlled trial randomized 200 patients with an average body mass index of 41 kg/m2 and AFib 1:1 to single-DCCV or dual-DCCV at three sites in Louisiana. The single-DCCV group received a 200J shock using a single set of defibrillator pads. The dual-DCCV group received synchronized shocks using two sets of defibrillator pads, totaling 400J. Failure of the first cardioversion was followed by a maximum of two additional dual-DCCV attempts in both groups.  Joshua Aymond, MD
Joshua Aymond, MD
The primary endpoint, successful cardioversion to sinus rhythm immediately following the first DCCV, occurred in 98% of patients in the dual-DCCV group compared to 86% of patients in the single-DCCV group (P=0.002). There were no adverse events in either cohort, including cardiovascular death or stroke. Moreover, there was no difference in chest discomfort.
“This is strong evidence to support using dual-DCCV as opposed to conventional single-DCCV in patients with a body mass index of 35 or higher who are undergoing cardioversion for atrial fibrillation,” said Joshua Aymond, MD, of Ochsner Health in New Orleans, and the study’s co-investigator.
Abelacimab demonstrated a highly significant reduction in major bleeding in patients with afib
Abelacimab offers the potential for safer and more effective hemostasis-sparing anticoagulation, according to the results of Abelacimab, a Novel Factor XI/XIa Inhibitor, Versus Rivaroxaban in Patients With Atrial Fibrillation: Primary Results of the AZALEA-TIMI 71 Randomized Trial.
Abelacimab is a novel, highly selective, fully human monoclonal antibody with a dual mechanism of action that locks factor XI(FXI) in the inactive state — preventing the formation of the activated FXI (FXIa).
Direct oral anticoagulants (DOACS) effectively prevent stroke in patients with AFib and are associated with low rates of life-threatening bleeding. Still, clinically significant bleeding frequently occurs, resulting in the undertreatment of patients.
The randomized, active-controlled trial conducted at 95 sites in the United States, Canada, Europe and Asia evaluated the safety and tolerability of two blinded doses of Abelacimab, 150 mg and 90 mg subcutaneous monthly, compared with open-label rivaroxaban in 1,287 patients with AFib at moderate to high risk of stroke.
Abelacimab, 150 mg and 90 mg, was associated with a 67% and 77% reduction in the primary endpoint, a composite of major or clinically relevant nonmajor bleeding, respectively. Both Abelacimab doses were associated with a 93% reduction in gastrointestinal bleeding, compared to rivaroxaban.
The trial, which was designed to continue until 166 patients experienced a primary endpoint, was stopped prematurely, after a median follow-up of 1.8 years, due to the overwhelming reductions in major and clinically relevant nonmajor bleeding. Abelacimab was well tolerated, with no statistically significant difference in ischemic or adverse events.
“AZALEA-TIMI 71 shows that factor XI inhibition, particularly with Abelacimab, is safer than the current DOACS we use for stroke prevention AFib,” said Christian Ruff, MD, MPH, director of general cardiology at Brigham and Women’s Hospital in Boston and senior investigator of the TIMI Study Group, the study’s principal investigator.
“It’s incredibly encouraging for the field, but because AZALEA-TIMI 71 wasn’t powered for efficacy, we are waiting for the results of definitive phase 3 trials that will hopefully demonstrate the efficacy of factor XI inhibition for stroke reduction."











